
Ncert Solution for 11 Class Chemistry Chapter 1 Some Basic Concepts of Chemistry
NCERT Solutions for Class 11 Chemistry PDF. Chapter 1 Some Basic Concepts of Chemistry. Chapter 2 Structure of Atom. Chapter 3 Classification of Elements and Periodicity in Properties. Chapter 4 Chemical Bonding and Molecular Structure. Chapter 5 Thermodynamics.

NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Basic Concepts of Chemistry
CBSE Class 11 Chemistry Chapter 1 NCERT Solutions. This Initial Chapter of Chemistry helps the students to understand the role played by Chemistry in different dimensions of life. Moving with the chapter, students will find details about the nature of material and chemical laws of combination. This very beginning of the chapter will help the.

Ncert Solution for 11 Class Chemistry Chapter 1 Some Basic Concepts of Chemistry
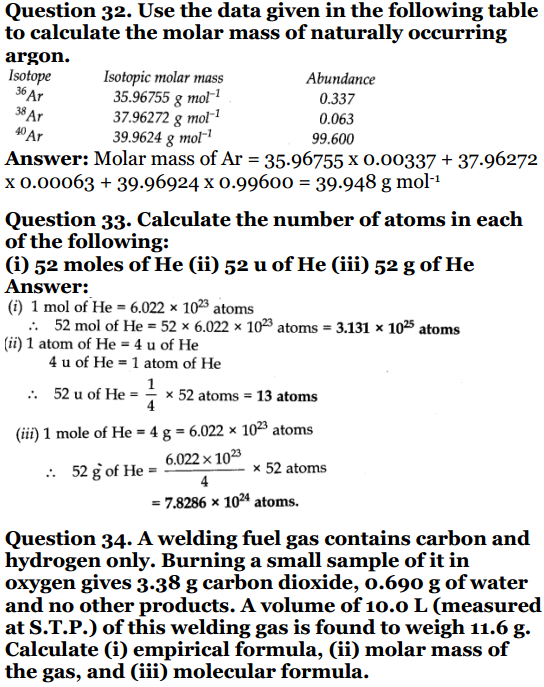
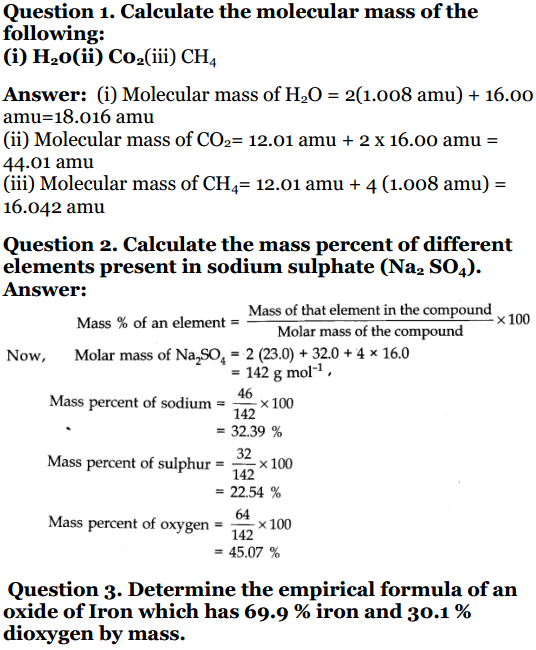
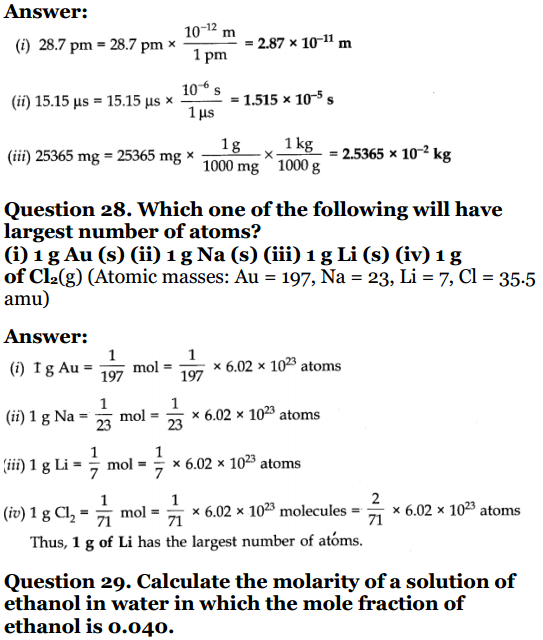
Question 1. Calculate the molecular mass of the following : (i) H 2 o (ii) CO 2(iii) CH 4Solution:(i) Molecular mass of H 2 O :2 × 1 + 1 × 16 = 18u (ii) Molecular mass of CO 2 :1 × 12 + 2× 16 = 44 u (iii) Molecular mass of CH 4 : 12 + 4 × 1 = 16 u Question 2. Calculate the mass percent of different elements present in sodium sulphate (Na 2 SO 4 ).
NCERT Solutions for Class 11 Chemistry (Updated for 2020 21)
The NCERT Solutions of Chemistry provided on this page for Class 11 Chapter 1 contains detailed explanations of the steps to be followed while solving the numerical value questions that are frequently asked in the examinations. The subtopics covered in the chapter are listed below.

Ncert Solution For Class 12 Chemistry Chapter 3 Electrochemistry
NCERT Solutions for Class 11 Chemistry. NCERT Solutions for Class 11 Chemistry in PDF format English Medium MCQ, Extra Questions for CBSE and State Board. As per the new textbook published for academic year 2023-24, there are only 9 chapters in class 11 chemistry Syllabus. Chapter 1. Some Basic Concepts of Chemistry. Chapter 2. Structure of Atom.

NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Click to Access Free PDF
Vedantu provides you with Class 11 Chemistry NCERT Solutions Chapter 1. Our expert professors of Chemistry explain the solutions of all questions as per the NCERT (CBSE) pattern. Some basic concepts of Chemistry Class 11 NCERT Solutions are given to make your study simplistic and enjoyable at Vedantu.

NCERT Books Free Download for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
NCERT Solutions For Chemistry Class 11 - Chapter at a Glance: A Quick Look at Key Concepts. The journey through CBSE Class 11 is an exciting and crucial phase in a student's academic life.
NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
In the NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry, a plethora of exercises are given so that students can understand the concepts as well as can solve confusions then and there.

Ncert Solutions For Class 11 Chemistry Chapter 1 Photos
NCERT solutions of class 11 Chemistry - Chapter List: • Chapter 1: Some Basic Concepts Of Chemistry. • Chapter 2: Structure Of Atom. • Chapter 3: Classification Of Elements And Periodicity In Properties. • Chapter 4: Chemical Bonding And Molecular Structure. • Chapter 5: States Of Matter Gases And Liquid. • Chapter 6: Thermodynamics.

NCERT Solutions for Class 11 Chemistry (Updated for 201920)
Here it is. NCERT Solutions for Class 11 Chemistry Chapter 1 PDF Download Link - Click Here to Download Solutions PDF How to download NCERT Solutions for Class 11 Chemistry Chapter 1 PDF? You can download the complete NCERT solutions for chapter 1 of this NCERT Book i.e. Chemistry Part I with following steps.

Diy Small Boat 500g, Wood Sailing Ship Model Kits Data, Ncert Solutions Class 10th Chemistry
on August 19, 2023, 10:59 AM. NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry in Hindi and English Medium exercises, MCQ and intext questions updated and modified for new academic session 2023-24. All the questions are as per new edition NCERT textbook published for 2023-24 exams.
NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
NCERT Solutions for Class 11 Chemistry Free PDF Download (Chapter-wise) Chapter 1 Some Basic Concepts of Chemistry Chapter 2 Structure of Atom Chapter 3 Classification of Elements and Periodicity in Properties Chapter 4 Chemical Bonding and Molecular Structure Chapter 5 States of Matter Chapter 6 Thermodynamics Chapter 7 Equilibrium

solution chemistry questions pdf
The NCERT Solutions of Chemistry given on this page for Class 11 Chapter 1 contains detailed ideas on the steps which have to be followed while solving the numerical value questions that are repeatedly asked in first term examinations. The subtopics covered in the chapter are given below.

NCERT Exemplar Class 11 Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
The chapter-wise links of NCERT Solutions for Class 11 Chemistry are as follows: Chapter-Wise NCERT Solutions for Class 11 Chemistry Chapter 1: Some Basic Concepts of Chemistry Chapter 2: Structure of Atom Chapter 3: Classification of Elements and Periodicity in Properties Chapter 4: Chemical Bonding and Molecular Structure
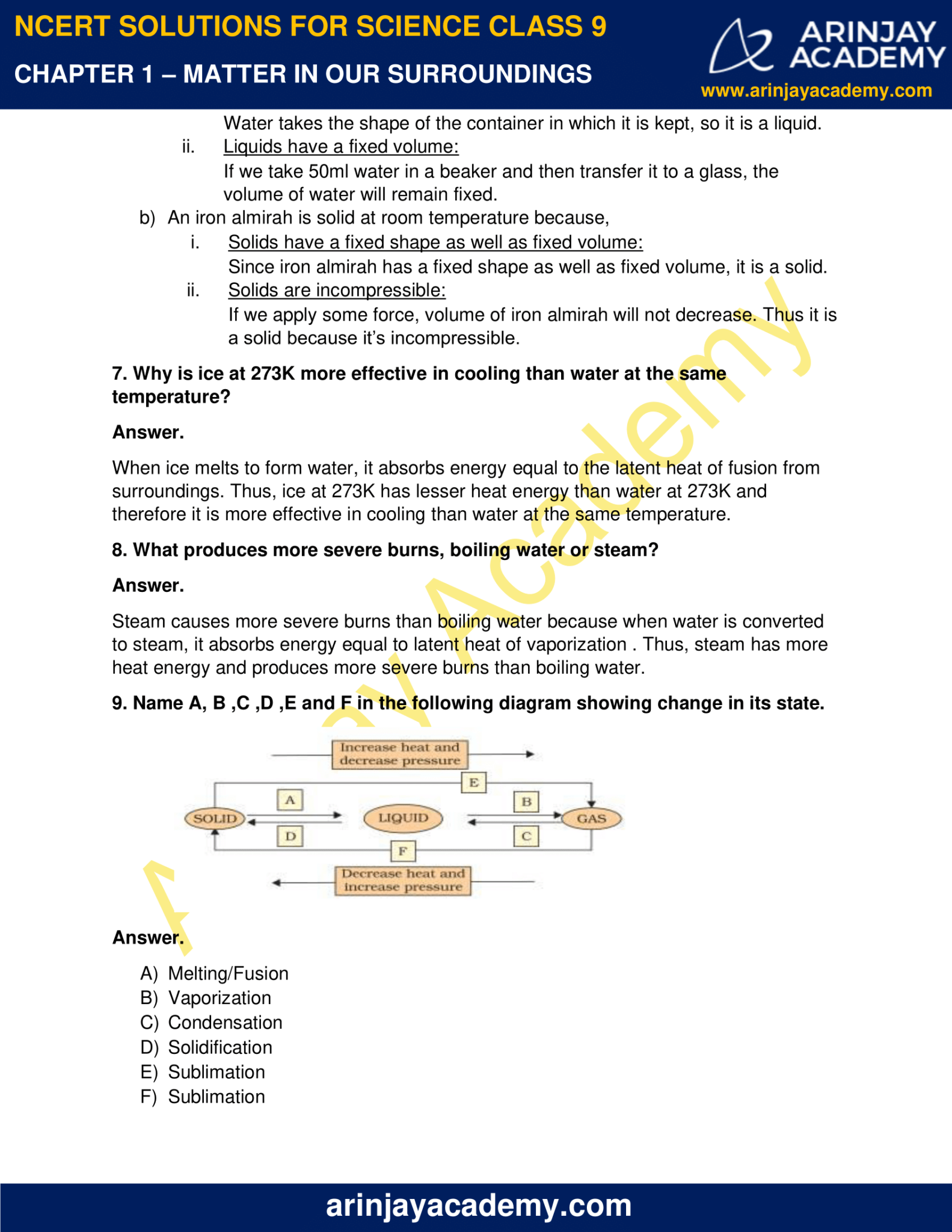
Ncert Book Class 9 Science Chapter 1 Matter In Our Surroundings —
Chapter 1 Some Basic Concepts of Chemistry Chapter 2 Structure of The Atom Chapter 3 Classification of Elements and Periodicity in Properties Chapter 4 Chemical Bonding and Molecular Structure Chapter 5 States of Matter Chapter 6 Thermodynamics Chapter 7 Equilibrium Chapter 8 Redox Reactions Chapter 9 Hydrogen Chapter 10 The sBlock Elements

NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
Solution : (i)CH4 : Molecular weight of methane, CH 4 = (1 x Atomic weight of carbon) + (4 x Atomic weight of hydrogen) = [1 (12.011 u) +4 (1.008u)] = 12.011u + 4.032 u = 16.043 u (ii) H2O : Molecular weight of water, H 2 O = (2 x Atomic weight of hydrogen) + (1 x Atomic weight of oxygen) = [2 (1.0084) + 1 (16.00 u)] = 2.016 u +16.00 u = 18.016u